- Uranium-Lead dating.
- online dating australia for free;
- The Age of the Earth.
- two hearts dating;
Retrieved 7 January Alpha-recoil in U-Pb geochronology: Effective sample size matters. Contributions to Mineralogy and Petrology , 4: Combined annealing and multi-step dissolution analysis for Improved precision and accuracy of zircon ages.
Navigation menu
Radiogenic Isotope Geology 2nd ed. Canon of Kings Lists of kings Limmu. Chinese Japanese Korean Vietnamese. Lunisolar Solar Lunar Astronomical year numbering.
Uranium–lead dating
Deep time Geological history of Earth Geological time units. Chronostratigraphy Geochronology Isotope geochemistry Law of superposition Luminescence dating Samarium—neodymium dating. Amino acid racemisation Archaeomagnetic dating Dendrochronology Ice core Incremental dating Lichenometry Paleomagnetism Radiometric dating Radiocarbon Uranium—lead Potassium—argon Tephrochronology Luminescence dating Thermoluminescence dating.
Fluorine absorption Nitrogen dating Obsidian hydration Seriation Stratigraphy.
Fundamentals of radiogenic isotope geology
Retrieved from " https: Wikipedia articles needing clarification from October Views Read Edit View history. This page was last edited on 16 January , at By using this site, you agree to the Terms of Use and Privacy Policy. Where is the time from starting point, the original amount of uranium, the amount of uranium at the measurement, the original amount of lead, the amount of lead at the measurement, the rate uranium changes to lead, the average rate of loss and gain in the amount of lead, the average rate of loss and gain in the amount of uranium. Uranium-Lead dating only works on igneous and metamorphic rocks because sedimentary layers contain small pieces of a other rock layers [3].
Uranium–lead dating - Wikipedia
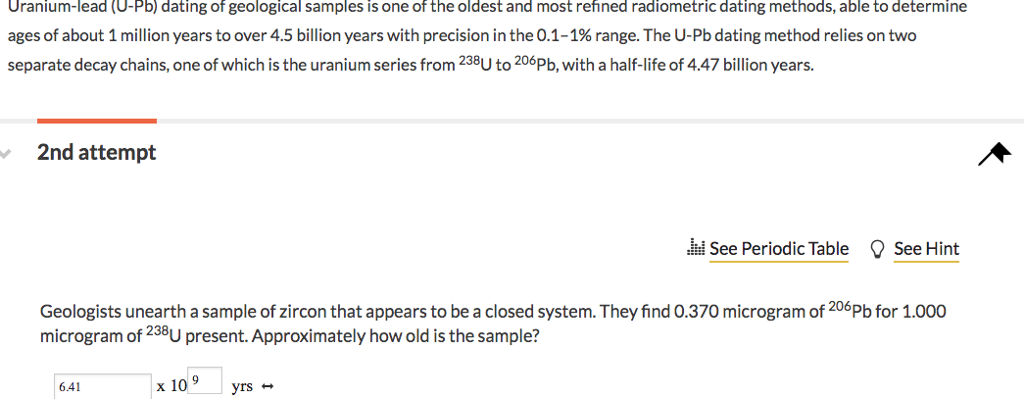
Like all radiometric dating methods, uranium-lead dating has a range that it works best. For uranium-lead has a range of 10 million to 4. This means that to begin with, any rock dated with this process will be in the 10's of millions [5]. For Uranium - Lead dating to work, scientists have to make three assumptions.
These assumptions are that the system being dated is a closed system ; at the beginning of the time period, there are no daughter isotopes present; and the rate of radioactive decay stays the same through the whole time period. Once all these assumptions are taken, the equation above simplifies to [4]. Without a closed system, uranium-lead dating, like all other radiometric dating methods, falls apart. Assuming a closed system means that nothing on the outside of the rock affected the sample. This means that none of the parent or daughter isotope leaked in or out.
It also implies that none of the factors that might affect the rate of the radioactive decay could not. This is an ideal concept that cannot happen.
If the ages this dating process generates are true, it gets harder to assume that nothing on the outside of the sample has any effect on the system. After a few million or billion years of a near-closed system, it will have a large error [6]. To find the age of a rock, a person trying to find it has to know the original amount of the parent isotope, and the original amount of the daughter isotope. The common assumption evolutionary scientists use is that the original amount was zero.
This is not scientific because at the beginning of that rock, there were no scientific observers to measure original amount of daughter isotope, in this case that would be lead and lead [4]. All radiometric dating systems depend on the idea that radioactive decay happens at a constant rate. It has been found that the rates fluctuate for an unknown reason.
One of the explanations has been found that the rates of decay of some radioactive isotopes change depending on the its proximity to the sun. Clearly, since half of the uranium has decayed to lead, and the half-life is 4. In the span of one half-life, one half of the uranium decayed to lead, and that requires 4.
Give an example of natural and artificial radioactivity? What is radioactivity and examples? Gimme an example of radioactivity we face in our daily lives? Explain how you determine the age of rocks by radioactivity. Answer Questions Why carbon molecules formed by covalent bonds are the most stable?
Identify the following reactions as double replacement, single replacement, composition, decomposition, acid-base, complex ion, anhydride.?
