Radioactive Dating
The natural log is just saying-- to what power do I have to raise e to get e to the negative k times 1. So the natural log of this-- the power they'd have to raise e to to get to e to the negative k times 1. Or I could write it as negative 1. That's the same thing as 1. We have our negative sign, and we have our k.
And then, to solve for k, we can divide both sides by negative 1.
- K-Ar dating calculation (video) | Khan Academy.
- dating a married leo man.
- .
- how long should you leave it before dating again.
- speed dating rating cards.
- K-Ar dating calculation?
- cool usernames online dating.
And so we get k. And I'll just flip the sides here. And what we can do is we can multiply the negative times the top. Or you could view it as multiplying the numerator and the denominator by a negative so that a negative shows up at the top. And so we could make this as over 1. Let me write it over here in a different color. The negative natural log-- well, I could just write it this way. If I have a natural log of b-- we know from our logarithm properties, this is the same thing as the natural log of b to the a power.
And so this is the same thing. Anything to the negative power is just its multiplicative inverse.
So this is just the natural log of 2. So negative natural log of 1 half is just the natural log of 2 over here.
5.7: Calculating Half-Life
So we were able to figure out our k. It's essentially the natural log of 2 over the half-life of the substance. So we could actually generalize this if we were talking about some other radioactive substance. And now let's think about a situation-- now that we've figured out a k-- let's think about a situation where we find in some sample-- so let's say the potassium that we find is 1 milligram. I'm just going to make up these numbers. And usually, these aren't measured directly, and you really care about the relative amounts.
But let's say you were able to figure out the potassium is 1 milligram. And let's say that the argon-- actually, I'm going to say the potassium found, and let's say the argon found-- let's say it is 0. So how can we use this information-- in what we just figured out here, which is derived from the half-life-- to figure out how old this sample right over here? How do we figure out how old this sample is right over there? Well, what we need to figure out-- we know that n, the amount we were left with, is this thing right over here.
So we know that we're left with 1 milligram.
And that's going to be equal to some initial amount-- when we use both of this information to figure that initial amount out-- times e to the negative kt. And we know what k is. And we'll figure it out later. So k is this thing right over here. So we need to figure out what our initial amount is.
We know what k is, and then we can solve for t. How old is this sample? We saw that in the last video. So if you want to think about the total number of potassiums that have decayed since this was kind of stuck in the lava. In a sample of rock that does not contain appreciable amounts of Pb, the most abundant isotope of lead, we can assume that lead was not present when the rock was formed.
Therefore, by measuring and analyzing the ratio of U Pb, we can determine the age of the rock.
Calculating Half-Life - Chemistry LibreTexts
This assumes that all of the lead present came from the decay of uranium If there is additional lead present, which is indicated by the presence of other lead isotopes in the sample, it is necessary to make an adjustment. Potassium-argon dating uses a similar method. K decays by positron emission and electron capture to form Ar with a half-life of 1.
If a rock sample is crushed and the amount of Ar gas that escapes is measured, determination of the Ar K ratio yields the age of the rock. Other methods, such as rubidium-strontium dating Rb decays into Sr with a half-life of As of , the oldest known rocks on earth are the Jack Hills zircons from Australia, found by uranium-lead dating to be almost 4.
Radioactive Dating
An ingenious application of half-life studies established a new science of determining ages of materials by half-life calculations. After one half-life, a 1. Present day estimates for the age of the Earth's crust from this method is at 4 billion years. Isotopes with shorter half-lives are used to date more recent samples.
Chemists and geologists use tritium dating to determine the age of water ocean and fresh. In addition, tritium dating can be useful in determining the age of wines and brandies. The half-life of an isotope is used to describe the rate at which the isotope will decay and give off radiation.
Radiometric dating
Using the half-life, it is possible to predict the amount of radioactive material that will remain after a given amount of time. Its half-life is approximately years. Skills to Develop Describe what is meant by the term half-life and what factors affect half-life. Calculate the amount of radioactive material that will remain after an integral number of half-lives. Calculate the age of a material based upon its half-life.
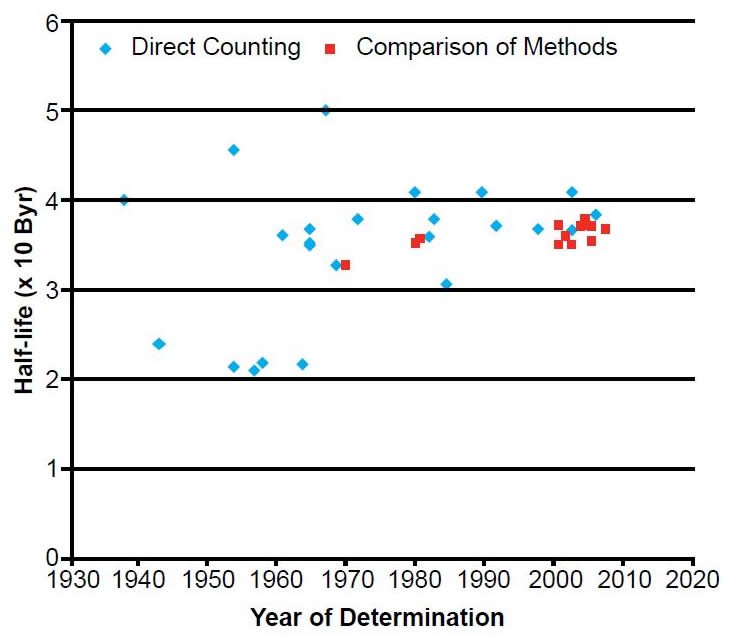
Describe how carbon is used to determine the age of carbon containing objects. Give examples of other isotopes used in radioactive dating. Appreciate the half-life of isotopes involved in nuclear weapons and reactors. The requirement of keeping the same number of nuclei gives.
Now suppose that there was an original amount of the daughter element present at the formation time of the sample being studied. This adds an additional unknown in the process, and requires an additional piece of data to permit a solution for elapsed time.
The requirement on the populations is now. Fortunately for radioactive dating processes, additional information is available in the form of other isotopes of the elements involved in the radioactive process. If there is another isotope of the daugther element D' which is presumed to be constant throughout the process, then the population requirement can be expressed in terms of the ratios. We can be reasonably confident that the isotope D' is contant if it is not radioactive not part of one of the natural radioactive series.
Using the radioactive decay equation as above, this becomes. Such a line is called an isochron since all the different minerals are presumed to have crystallized together and therefore have the same age since solidification. The age can then be calculated from that slope as follows:.
.jpg)