That is pretty accurate!!! Other events on earth can be dated equally well given the right minerals.
Age of the Earth - Wikipedia
For example, a problem I have worked on involving the eruption of a volcano at what is now Naples, Italy, occurred years ago with a plus or minus of years. Yes, radiometric dating is a very accurate way to date the Earth. We know it is accurate because radiometric dating is based on the radioactive decay of unstable isotopes. For example, the element Uranium exists as one of several isotopes, some of which are unstable. When an unstable Uranium U isotope decays, it turns into an isotope of the element Lead Pb.
We call the original, unstable isotope Uranium the "parent", and the product of decay Lead the "daughter". From careful physics and chemistry experiments, we know that parents turn into daughters at a very consistent, predictable rate. A geologist can pick up a rock from a mountainside somewhere, and bring it back to the lab, and separate out the individual minerals that compose the rock. They can then look at a single mineral, and using an instrument called a mass spectrometer, they can measure the amount of parent and the amount of daughter in that mineral.
The ratio of the parent to daughter then can be used to back-calculate the age of that rock. The reason we know that radiometric dating works so well is because we can use several different isotope systems for example, Uranium-Lead, Lutetium-Halfnium, Potassium-Argon on the same rock, and they all come up with the same age.
4.55 ± 0.05 Gyr
This gives geologists great confidence that the method correctly determines when that rock formed. Hope that helps, and please ask if you'd like more details! I think that I will start by answering the second part of your question, just because I think that will make the answer to the first question clearer. Radiometric dating is the use of radioactive and radiogenic those formed from the decay of radioactive parents isotopes isotopes are atoms of the same element that have different numbers of neutrons in their nuclei to determine the age of something.
The Idea of Time
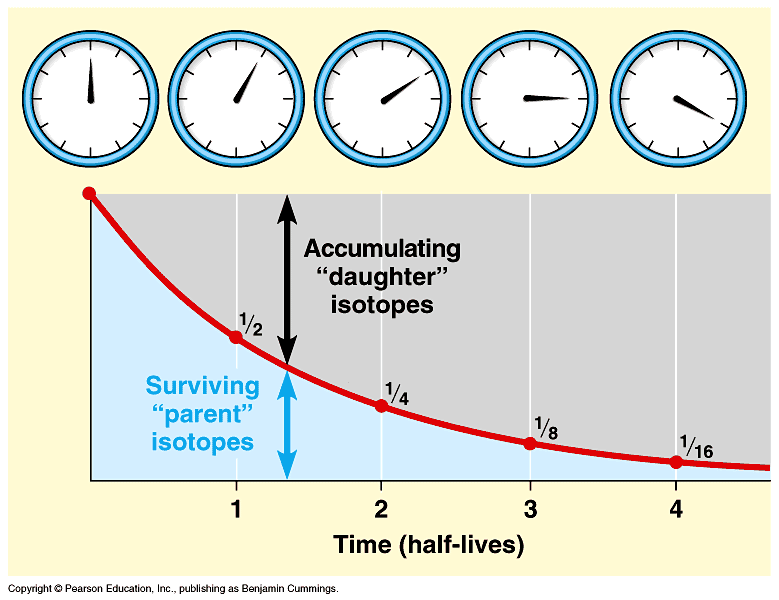
It is commonly used in earth science to determine the age of rock formations or features or to figure out how fast geologic processes take place for example, how fast marine terraces on Santa Cruz island are being uplifted. Radiometric dating relies on the principle of radioactive decay. All radioactive isotopes have a characteristic half-life the amount of time that it takes for one half of the original number of atoms of that isotope to decay.
By measuring the parent isotope radioactive and the daughter isotope radiogenic in a system for example, a rock , we can tell how long the system has been closed in our example, when the rock formed. The process of radiogenic dating is usually done using some sort of mass spectrometer.
A mass spectrometer is an instrument that separates atoms based on their mass. Because geochronologists want to measure isotopes with different masses, a mass spectrometer works really well for dating things. I do think that radiometric dating is an accurate way to date the earth, although I am a geochronologist so I have my biases. Most estimates of the age of the earth come from dating meteorites that have fallen to Earth because we think that they formed in our solar nebula very close to the time that the earth formed.
The fact that the age we calculate is reproducible for these different systems is significant. We have also obtained a very similar age by measuring Pb isotopes in materials from earth. I should mention that the decay constants basically a value that indicates how fast a certain radioactive isotope will decay for some of these isotope systems were calculated by assuming that the age of the earth is 4. The decay constants for most of these systems have been confirmed in other ways, adding strength to our argument for the age of the earth.
Radiometric dating depends on the chemistry and ratios of different elements. It works like this:. Take, for example, zircon, which is a mineral; its chemical formula is ZiSiO 4 , so there is one zirconium Zi for one silicon Si for four oxygen O. One of the elements that can stand in chemically for zircon is uranium.
Uranium eventually decays into lead, and lead does not normally occur in zircon, except as the radioactive decay product of uranium. Therefore, by measuring the ratio of lead to uranium in a crystal of zircon, you can tell how much uranium there originally was in the crystal, which, combined with knowing the radioactive half-life of uranium, tells you how old the crystal is.
Obviously, if the substance you are measuring is contaminated, then all you know is the age since contamination, or worse, you don't know anything, because the contamination might be in the opposite direction - suppose, for example, you're looking at radio carbon carbon 14, which is produced in the atmosphere by cosmic rays, and which decays into nitrogen. Our planet was pegged at a youthful few thousand years old by Bible readers by counting all the "begats" since Adam as late as the end of the 19th century, with physicist Lord Kelvin providing another nascent estimate of million years.
Kelvin defended this calculation throughout his life, even disputing Darwin's explanations of evolution as impossible in that time period.
- dating and marriage customs in canada.
- new dating site in germany.
- dating advice with lucas.
- How is Earth's Age Calculated?.
- Age of the Earth.
- .
- How is Earth's Age Calculated?.
In , Marie Curie discovered the phenomenon of radioactivity, in which unstable atoms lose energy, or decay, by emitting radiation in the form of particles or electromagnetic waves. By physicist Ernest Rutherford showed how this decay process could act as a clock for dating old rocks.
- african dating ghana.
- bahai dating sight.
- speed dating capital.
- aunties phone numbers dating in hubli.
- UCSB Science Line.
Meanwhile, Arthur Holmes was finishing up a geology degree at the Imperial College of Science in London where he developed the technique of dating rocks using the uranium-lead method. By applying the technique to his oldest rock, Holmes proposed that the Earth was at least 1. In a report of his findings published in in the journal Nature , Holmes expressed the less-than-ecstatic reception his findings received: Since then, several revisions have been made.
In the s, Earth's age crept up toward 3 billion years, making it for a time even older than the universe, which was then estimated to be about 1.