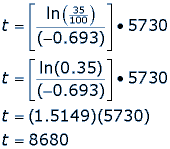By other non-scientific estimates I'd put it to years old. Would it be possible to use Radiocarbon C14 dating to get a good estimate? I've seen C-dating used for ancient samples BC etc. Of course, one must assume the wood was used not too long after it was cut down I suppose.
- Radiometric dating?
- ChemTeam: Half-life problems involving carbon?
- ?
- good dating apps for college students.
Sample amount is not a problem: This question does not appear to be about physics within the scope defined in the help center. If this question can be reworded to fit the rules in the help center , please edit the question. This is enough information to calculate the age of any organic, or living material, up to an age of around 40 to 50 years i. For your situation, all that is known is the half-life of carbon Hope this helps, I know I've repeated things that you know but in case there's anything you missed.
If you want to go even further back, you can look at cave deposits, and the fancy word for these cave deposits are speleothems. You might be familiar with stalagmites.
Radiocarbon Dating - Chemistry LibreTexts
Those are those speleothems that are kind of coming out of the bottom of the cave, or stalactites. Those are the speleothems that are coming from the top of the cave. But the reason why these are useful is these are formed by calcium carbonate, so they have carbon in them, and slowly over, really, tens of thousands of years, the water in the cave deposits that calcium carbonate.
So it's a record of the fraction of carbon in some of those years. And you can go down to resolutions of as small as 10 years.
How do you calculate half life of carbon 14?
And so this will give us pretty good estimates over tens of thousands of years, up to 50, years. And frankly, carbon isn't even useful beyond, really, 50, or 60, years. So this gives us a good record of carbon in the atmosphere, assuming that it's fairly uniform throughout the atmosphere, and all evidence suggests that, and that that uniformity through the atmosphere also goes into the water supply, and into living plants and animals. If, however, your goal is to determine the half life of carbon so you can use it to determine an age, then Google is your friend: How do you calculate half life of carbon 14?
The formula for half life calculations is: To do this, we need to use logarithms: Related questions Why does carbon 14 undergo radioactive decay?
How can half-life be described in terms of radioactive decay? What are some examples of radioisotopes?